Canadian clinical-stage immunotherapy company Medicenna Therapeutics Corp. has outlined a significant and potentially transformative roadmap for 2026, buoyed by encouraging clinical data for its lead asset and planned advancements for a novel therapeutic candidate.
MDNA11 Demonstrates Impressive Monotherapy Activity
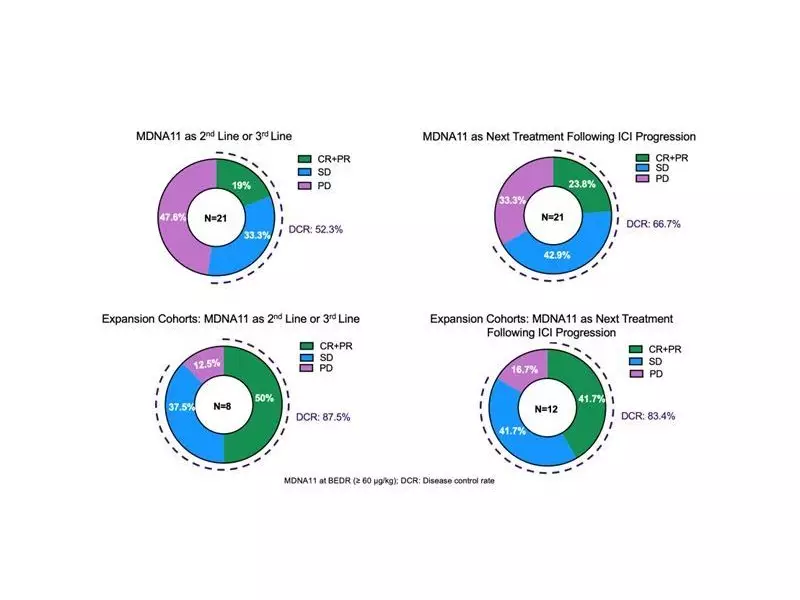
The company provided key updates from its ongoing ABILITY-1 Phase 1/2 study of MDNA11, a superkine designed as an IL-2 super agonist. In specific monotherapy expansion cohorts involving 21 patients, the therapy showed notable response rates. Among patients treated in the second- or third-line (2L/3L) setting, the objective response rate (ORR) was an impressive 50%.
Perhaps more striking was the performance in patients whose last treatment was an immune checkpoint inhibitor (ICI) that had failed. In this challenging patient population with limited options, MDNA11 achieved an ORR of 42% as the next line of therapy. Medicenna management believes this data underscores the drug's best-in-class potential for advanced cancer patients who have exhausted other treatments.
When looking at a broader group of 55 efficacy-evaluable monotherapy patients across 18 different cancer types, the results remained promising. The ORR was 19% for MDNA11 as a 2L/3L treatment and 24% when administered after ICI failure.
Strategic Clinical Path Forward and New Studies
Based on this accumulating data, Medicenna has outlined clear next steps. The company plans to complete enrollment for the ABILITY-1 Phase 1/2 trial and is preparing for a registrational trial focused on patients with 2L/3L melanoma and select other tumor types following ICI therapy failure.
Furthermore, the therapy will be evaluated in a new front-line setting. The NEO-CYT study, sponsored by Fondazione Melanoma Onlus, will assess MDNA11 as a front-line therapy for patients with resectable advanced cutaneous melanoma. Patient enrolment for this study is scheduled to commence in the first half of 2026, with interim data anticipated in the second half of the year.
Pipeline Expansion with MDNA113 and Financial Runway
Looking beyond MDNA11, Medicenna is advancing its pipeline with MDNA113, a novel, targeted bifunctional superkine. This candidate is designed as a conditionally activated fusion of a best-in-class IL-2 and an anti-PD1 antibody. Preclinical data in non-human primates has shown a favourable safety profile at the highest tested dose of 30 mg/kg, prospectively supporting a human dosage comparable to existing anti-PD-1 therapies.
The company believes MDNA113's unique design will allow it to excel against other bifunctional programs in development. An Investigational New Drug (IND) submission and the initiation of a first-in-human trial for MDNA113 are expected in the second half of 2026.
Medicenna also updated its financial guidance, stating it has a cash runway extending into the third quarter of calendar 2026, providing the resources to execute these plans. The company, which trades on the TSX and OTCQX, will also present data related to its bizaxofusp program at a glioblastoma summit in February 2026.
Fahar Merchant, PhD, President and CEO of Medicenna, stated that the company is building on 2025's milestones and is poised for a transformative year. He emphasized the goal of establishing Medicenna as a leader in superkine-based therapeutics, leveraging its platforms to advance precise immunotherapies for 'cold' tumors unresponsive to current treatments.