Novo Nordisk Escalates Legal Battle Against Hims & Hers Over Obesity Drug Copycats
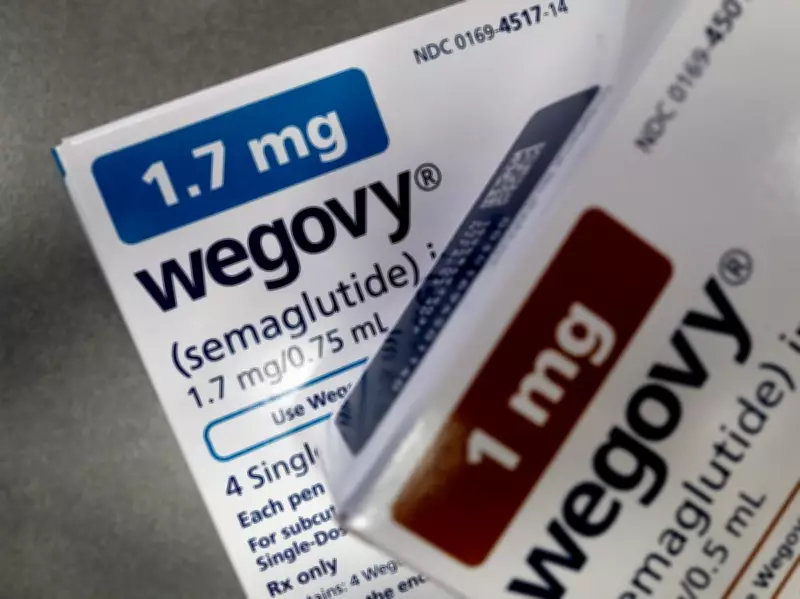
In a significant escalation of corporate tensions, Novo Nordisk A/S has filed a lawsuit against Hims & Hers Health Inc. for allegedly producing and marketing copycat versions of its blockbuster obesity medications. This legal action comes despite Hims & Hers recently scrapping plans to sell a knock-off version of Novo's Wegovy pill, highlighting the increasingly acrimonious relationship between the two healthcare companies.
Patent Infringement Allegations
Novo Nordisk argues that Hims & Hers is breaching United States patents on semaglutide, the active ingredient in Novo's highly successful obesity treatments Wegovy and Ozempic. The Danish pharmaceutical giant filed the lawsuit on Monday, targeting not only Hims' now-abandoned plans for a copycat pill but also existing shots that mimic Wegovy and Ozempic formulations.
This represents a strategic shift for Novo Nordisk under chief executive Mike Doustdar, marking the first time the company has pursued legal action specifically for patent infringement related to compounded semaglutide. Previously, Novo's legal approach focused primarily on how companies marketed their products rather than direct patent challenges.
Regulatory Scrutiny and Market Reactions
The lawsuit follows a turbulent period for Novo Nordisk that included regulatory scrutiny from the U.S. Food and Drug Administration. In a letter dated February 5th, the FDA criticized a television advertisement for Novo's new weight-loss pill, calling claims about its effectiveness "false or misleading." Novo spokesperson Liz Skrbkova confirmed the company is addressing the FDA's concerns regarding the advertisement's presentation.
Market reactions to these developments have been dramatic. Hims & Hers shares plummeted 24 percent in New York trading, representing their steepest decline in nearly eight months. Meanwhile, Novo Nordisk stock initially surged as much as nine percent on Monday following Hims' decision to scrap the Wegovy pill copycats, but those gains moderated after news of both the lawsuit and FDA letter emerged.
Escalating Corporate Conflict
The legal action intensifies an ongoing dispute between the companies that began when they terminated their partnership last year. According to Novo Nordisk general counsel John Kuckelman, Hims' decision to launch a knock-off Wegovy pill represented "a step too far" and served as "a tipping point" in their deteriorating relationship.
Novo Nordisk maintains that its medications are produced in strict compliance with FDA requirements and safety controls, contrasting them with what the company characterizes as inferior Hims products. In response, Hims & Hers issued a statement calling the lawsuit "a blatant attack by a Danish company on millions of Americans who rely on compounded medications for access to personalized care."
The telehealth company further accused "Big Pharma" of weaponizing the U.S. judicial system to limit consumer choice and pledged to continue fighting for "choice, affordability, and access" in the healthcare market.
Broader Industry Context
This legal confrontation occurs against a backdrop of intense competition in the obesity drug market. Novo Nordisk recently issued a conservative sales forecast for the year, contrasting sharply with rival Eli Lilly & Co.'s more ambitious expectations. The market for weight-loss medications has become increasingly competitive as pharmaceutical companies race to develop effective treatments for obesity, a condition affecting millions of Canadians and Americans.
The outcome of this lawsuit could have significant implications for how pharmaceutical companies protect their intellectual property in the rapidly evolving telehealth and compounded medication sectors. As both companies prepare for a potentially lengthy legal battle, healthcare consumers and investors will be watching closely to see how this conflict shapes access to obesity treatments and competitive dynamics in the pharmaceutical industry.