Complaint Prompts Recall of Birth Control Pills in Canada
A recent complaint has triggered a significant recall of birth control pills prescribed across Canada, raising alarms over potential health risks due to improper packaging. The recall, initiated by Teva Canada Inc., comes after reports of missing pills in some packages, which could compromise contraceptive effectiveness and lead to unintended pregnancies.
Details of the Recall Notice
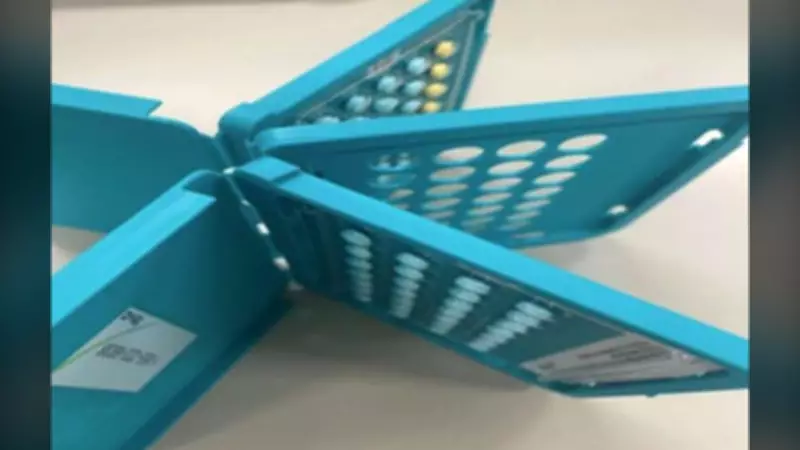
Teva Canada Inc. issued a recall notice that includes a handout image showcasing an example of the defective packaging. The image illustrates how certain packages may lack the full complement of birth control pills, a critical issue that undermines the reliability of these medications. This recall underscores the importance of stringent quality control in pharmaceutical manufacturing, especially for products that impact reproductive health.
The complaint that sparked this action was filed by a healthcare professional or consumer who noticed discrepancies in the packaging. Such vigilance highlights the role of public and professional oversight in maintaining drug safety standards. Health authorities are urging patients to check their medication packages and consult with pharmacists or doctors if they suspect any irregularities.
Health Implications and Consumer Advice
Missing birth control pills pose serious health risks, including reduced contraceptive efficacy and potential hormonal imbalances. Patients relying on these pills for family planning or medical conditions may face unintended consequences if they consume incomplete doses. It is crucial for individuals to verify their pill counts and report any issues immediately to healthcare providers.
Teva Canada has advised consumers to return affected products to pharmacies for replacement or refund. The company is working closely with Health Canada to investigate the root cause of the packaging errors and implement corrective measures. This incident serves as a reminder for pharmaceutical firms to adhere to rigorous packaging protocols to prevent similar occurrences in the future.
Broader Context and Regulatory Response
This recall is part of a broader trend of drug safety concerns in Canada, where regulatory bodies like Health Canada actively monitor and respond to complaints. The swift action taken in this case demonstrates the effectiveness of the complaint-driven system in safeguarding public health. However, it also raises questions about the overall quality assurance processes within the pharmaceutical industry.
Experts emphasize that such recalls can erode public trust in medication safety, making it imperative for companies to prioritize transparency and accountability. Patients are encouraged to stay informed about drug recalls through official channels and to participate in reporting any adverse effects or packaging defects they encounter.
In conclusion, the recall of birth control pills in Canada, prompted by a complaint over packaging issues, highlights ongoing challenges in drug safety and quality control. Consumers should remain vigilant, while regulators and manufacturers must collaborate to ensure that medications meet the highest standards of reliability and safety.





