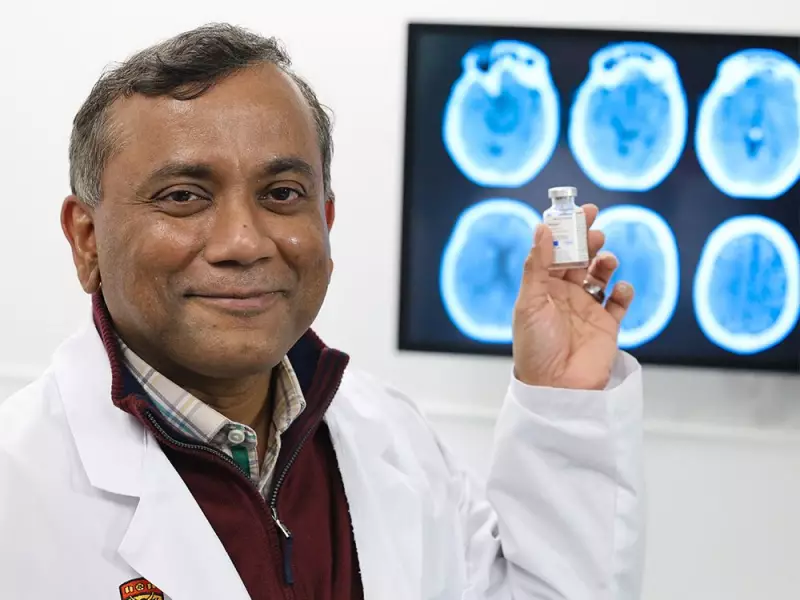
A groundbreaking study from the University of Calgary has directly influenced worldwide medical guidelines for treating strokes, introducing a faster, more efficient drug that is being hailed as a significant advancement in emergency care.
A Revolutionary Shift in Stroke Treatment
The research, led by neurologist and researcher Dr. Bijoy Menon, compared the established drug Alteplase with Tenecteplase (TNK) for acute ischemic stroke. The findings were decisive: Tenecteplase proved far superior in administration speed, transforming the initial response to a life-threatening event.
"With strokes, just as with heart attacks, every minute counts," emphasized Dr. Menon, the study's principal investigator and head of neurology at the Cumming School of Medicine. "If you’re delaying the giving of the drug, or not giving it efficiently, it can affect patient outcomes."
From an Hour to Five Seconds
The previous standard, Alteplase, required a complicated infusion process taking more than 60 minutes to administer. The new protocol using Tenecteplase slashes that time dramatically.
"This is a drug we can give just over five seconds" using a standard intravenous (IV) line, Menon stated. This ease of use is particularly transformative for stroke patients who first arrive at smaller, rural hospitals before transfer to major stroke centres.
"Now that we have this drug, transport itself has become easy and less complicated," Menon explained. "We don’t have to have these complex infusion pumps in ambulances... We can now give the drug and immediately transport the patient to the big hospitals."
A Landmark Canadian Clinical Trial
The University of Calgary-led investigation stands as the largest acute stroke clinical trial in Canada. It involved:
- 1,600 patients across the country.
- 22 research sites nationwide.
- Conducted over two years during the COVID-19 pandemic.
The pivotal findings were published in the prestigious Lancet medical journal in 2022. The study was conducted as part of the Calgary Stroke Program, a collaborative team of physicians, nurses, and engineers dedicated to advancing stroke care.
Notably, this is the first University of Calgary investigator-initiated trial to receive approval from the U.S. Food and Drug Administration (FDA). This regulatory endorsement is a gold standard, often involving FDA inspectors visiting the research team to verify the high-quality methodology.
"When you do academic investigator-initiated trials, you do it because you want to test a hypothesis, but when you’re doing it from a regulatory perspective, everything else needs to be at a very high standard," Menon noted, highlighting the study's rigorous validation.
The global adoption of these new guidelines, spurred by the Calgary research, means that stroke patients everywhere will benefit from a treatment that is not only effective but astonishingly swift, turning minutes saved into brain function preserved.





